INTERCER: EXPERTS IN INTERNATIONAL CERTIFICATION
Intercer has international agreements with entities specialized in the pharmaceutical and health sector, which allows services to the Spanish and European Industry, in order to break the technical barriers that limit the movement of products and services around the world.
Some examples of these services are:
MÉXICO SAMPLE:


REQUIREMENTS FOR THE PROCESS OF SANITARY REGISTRATION OF MEDICAL DEVICES
Homoclaves: COFEPRIS-04-001-A, COFEPRIS-04-001-B and COFEPRIS-04-001-C
Class III Medical Devices in accordance with Art. 83 of the Health Supplies Regulation
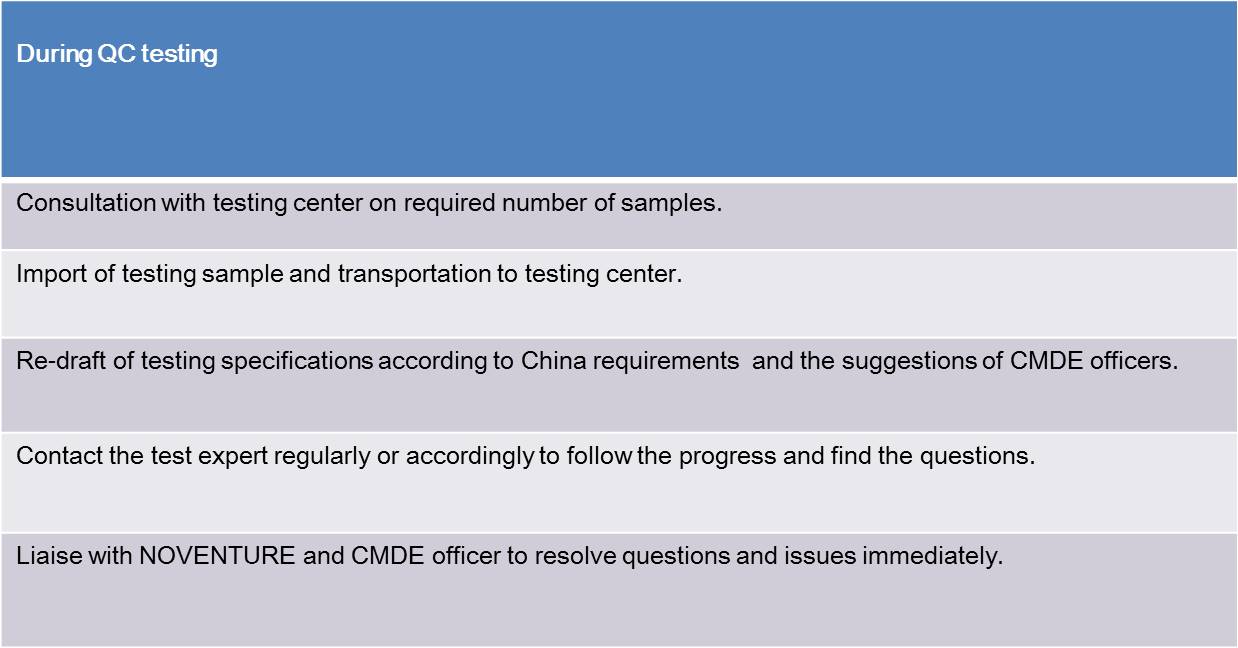
PRODUCT REGISTRATION IN THE PEOPLE'S REPUBLIC OF CHINA


TRUST
SECURITY
FLEXIBILITY
Certification, inspection and audit solutions focused on business optimization.
AREAS DE ACTIVIDADES INTERCER
Copyright INTERCER. All rights reserved.
NOTA: ESTA WEB NO UTILIZA COOKIES NI NINGÚN MEDIO DE CONTROL VISITANTES.